Thousands of foods have allergic reactions associated with them, and in theory, it’s possible for any food to be an allergy candidate.
However, the actual part of the food we can potentially be allergic to is the proteins and their pollens.
Not all foods have those, or at least not in high concentrations.
Can you be allergic to apples? Yes, but since they contain so little protein content, even if you are allergic, it’s unlikely you will have noticeable side effects like skin rash, hives, or sneezing.
Among those eaten, Gala apples have the least amount of protein, and Starking has the most (1). In skin prick tests, that’s why the latter variety tends to have more pronounced results.
[toc]
- Milk
- Eggs
- Fish
- Crustacean shellfish
- Tree nuts
- Peanuts
- Wheat
- Soybeans
Many within these categories are also foods high in lectins. Is that just a coincidence, or does their presence plays a part in symptoms?
It’s possible that your side effects might not even be from a soy, nut, or dairy allergy. They might be caused by your having an increased sensitivity to a specific form of lectin poisoning.
The most common thing you’ve never heard of
You could spend a whole year reading nutritional websites yet never come across this word once.
Or at least until recently.
Now you may encounter an ad to sell you the Lectin Shield supplement by Gundry MD.
To complement the narrative, Dr. Gundry is also the author of the much-hyped book called The Plant Paradox: The Hidden Dangers in “Healthy” Foods. Kelly Clarkson has touted it.
It seems all you hear on the topic is scaremongering you into buying expensive supplements.
Or someone independent who reports on what Gundry says as gospel.
How about some unbiased insight from other sources?
Lectin intolerance and toxicity affects all of us to at least some degree.
Lectins were discovered almost 130 years ago, in 1888. That’s well over a decade before aviation, something we have since mastered. Yet these commonly occurring compounds largely remain a mystery. And while you may not be flying every day, it’s guaranteed that you are eating foods containing lectin on a daily basis.
Seems like something we really ought to know more about, right?
Glyca-binding proteins (GBPs) are a category of proteins that bind specifically to certain sugar molecules. The “glyca” is the same prefix you see in the word glycation. That describes what happens after a protein or fat binds with a sugar molecule (3).
This binding process can cause inflammation and the creation of advanced glycation end products (AGEs), which are compounds associated with numerous age-related diseases. It’s why the apparent anti-glycation benefits of carnosine are so intriguing.
While some is influenced by diet, most of the glycation in your body will take place no matter what you eat.
There are two major categories of GBPs:
- lectins
- glycosaminoglycan-binding proteins
That last one is more relevant to pharmaceuticals (like heparin, a blood thinning medication) and how some infectious diseases work (like Streptococcus) (4). Since we’re focusing on food here, this second category will have to be a conversation for another time.
What are lectins?
Lectins are a defense mechanism which all life forms appear to have. Essentially, they are a low level toxin. The purpose of lectins is to discourage other animals from eating that life form. By triggering a negative reaction in the predator, that life form is then viewed as an undesirable food source. Hence, aiding its future survival.
Other names for lectins include agglutinins and hemagglutinins. When it comes to animal lectins, they’re almost always referred to as either agglutinins, glycans, glycan-binding proteins, or glycoproteins.
That’s why if you do a search, almost all of what you will see is related to plant foods.
As part of an avoidance diet, many folks are peddling a lectin-free food list (or so they believe) by axing many grains, vegetables, and fruits. They have suggested intolerance of them is causing weight gain, inflammation, leaky gut, and even major diseases.
That might be happening with some. But only picking on plants is premature.
Does meat like beef, chicken, and turkey have lectins? Yep…
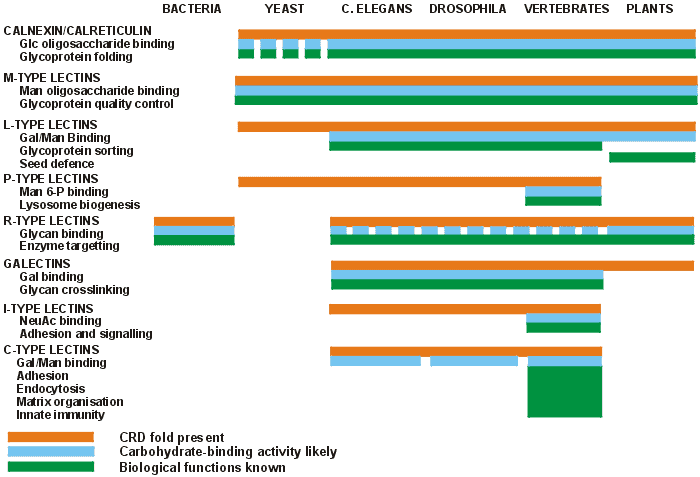
Not only do meats (vertebrate animals) have the same broad types/categories as plants, but they also have others in addition, as seen above.
Whether you’re talking about meat, dairy, or eggs, those coming from animals are some of the least studied and most recent to be discovered.
What we do know, though, is that many of them bind not just to carbs but also proteins and fats (6). That means overall, the animal forms may actually have a greater biological effect on the human body than those which are plant-derived.

Mammals only produce milk during reproduction to feed their newborns.
Since reproduction is an animal’s most important task in life, it’s interesting to note that it also involves their most significant use of lectins – the mother’s milk is loaded with it.
This fends off predators – other species – from considering that milk as their own potential food source. That ensures the newborn is more likely to get this vital source of food rather than someone else.
Keep in mind that predators include microscopic organisms, like bad bacteria and fungal infections, in a baby’s digestive tract.
So why are these milk lectins nutritious for the baby while being potentially poisonous to another species?
That’s how lectins work. They ensure the food is good for whoever should get it, but not good for everyone.
Is lectin the same as lecithin? No, they sound similar but are completely different things. While the former is a sugar-binding protein, the latter – lecithin – is a generic term for a broad category of yellowish-brown lipids (fats) found in plants and animals.
What types of food contain the most lectins?

That’s not a straightforward answer since each plant and animal has a unique type (or several).
However, if you were treating them all as equal and simply basing your answer on percentage concentrations, these groups represent some of the highest natural sources identified so far.
- Nightshade fruits and vegetables – Such as white potatoes, tomatoes, eggplants, and peppers. Goji berries are also part of the Solanaceae family, which means they’re nightshade.
- Grains – Whole grains have more since they are found in higher concentrations in the outer bran.
- Dairy – Pasteurized milk and dairy products may be worse because the heat destroys the naturally present SIgA, an immunoglobulin that may help neutralize lectins to some degree by binding to them (7).
- Legumes – Beans, lentils, and peas.
- Nuts and seeds – Excluding grain seeds, there is not much evidence to suggest that humans are sensitive to the types of lectins in tree nuts and most flowering seeds.
- Yeast – It’s worth noting that “only a few reports are available” about the activity of yeast lectins (8).
Just because you don’t see food on the list doesn’t mean it’s safe.
Scientists have only recently concluded that they are found in every plant and animal.
In fact, scientists have yet to identify a single organism, whether that’s something as large as an animal or as small as an insect or bacteria, which does not produce glycan-binding proteins. They find them whenever they have looked for them in a given species.
For example, research published in 2015 found them in dozens of tested fish, including those we commonly eat, like salmon, catfish, trout, tilapia, mackerel, and even eels (9).
Grains, nightshades, and dairy have long been scrutinized as being the worst. However that might be a premature conclusion. Scientists initially focused on those because their percentage content was obviously high. But less doesn’t necessarily mean better, since the types of lectin differ by food. Most have little to no research behind them.
The current mindset might be comparable to assuming a big truck full of dynamite is more dangerous than a small suitcase-sized nuclear bomb.
Quantity aside, the unique effects of each type need to be evaluated.
To give you an idea of their diversity, here is a table of lectins that have been discovered. These 50 are just a sampling of the thousands – perhaps tens of thousands – in our diet, most of which are unknown/unstudied.
With the exception of dairy and wheat, many foods have only had one type researched, even though they may contain several.
List of 50 Foods High in Lectins(list in alphabetical order) |
|||
|---|---|---|---|
| Food | Lectin Name* | Abbreviation** | |
| 1. | Avocado | Persea americana agglutinin | PAA |
| 2. | Bananas | Plantain agglutinin | none assigned (BanLec is shortened name) |
| 3. | Barley | Hordeum vulgare agglutinin | HOV |
| 4. | California crab | Cancer antennarius agglutinin | CCA |
| 5. | Carrot | Daucus carota agglutinin | DAC |
| 6. | Chickpea | Cicer arietinum agglutinin | CPA |
| 7. | Cocoknut crab | Birgus latro agglutinin | BIL |
| 8. | Corn | Zea mays agglutinin | ZMA (corn lectin is more often used) |
| 9. | Eggplant | Solanum melongena agglutinin | SOM |
| 10. | Elderberry | Sambuccus nigra agglutinin | SNA |
| 11. | Fava bean | Vicia faba agglutinin | VFA |
| 12. | Garlic | Allium sativum agglutinin | ASA |
| 13. | Gourd | Luffia actangula agglutinin | LUA |
| 14. | Jackfruit | Artocarpus integrifolia agglutinin (Jacalin) | JAC |
| 15. | Kidney bean | Phaseolus vulgaris agglutinin | PHA |
| 16. | Leek | Allium porrum agglutinin | APA |
| 17. | Lentil | Lens culinaris agglutinin | LCA |
| 18. | Lobster | sialic acid-specific lobster lectin | LAg1 |
| 19. | Mango | Mangifera indica agglutinin | MIH |
| 20. | Milk (mammals, incl. cow) | A1 beta-casein (beta-casomorphin-7) | BCM-7 (name of 7 amino acid segment unique to A1) |
| 21. | Milk (mammals, incl. cow) | A2 beta-casein | none assigned |
| 22. | Milk (mammals, incl.cow) | phosphorylated whey glycoprotein | PP3 |
| 23. | Milk (mammals, incl. cow) | chitinase-3-like protein 1 (chitinase-like lectin) | Chi3L1 & YKL-40 |
| 24. | Milk (mammals, incl. cow) | pregnancy-associated glycoproteins | PAGs (numerous subtypes) |
| 25. | Mung bean | Vigna radiata agglutinin | VRA |
| 26. | Mushroom | Agaricus bisporus agglutinin | ABA |
| 27. | Mushroom | Psathyrella velutina agglutinin | PSV |
| 28. | Oat | Avena sativa agglutinin | AS |
| 29. | Onion | Allium cepa agglutinin | ACA |
| 30. | Pea | Pisum sativum agglutinin | PSA |
| 31. | Peanut | Peanut agglutinin | PNA |
| 32. | Peppers (incl. sweet bell and hot chili peppers) | Capsicum annuum GLP1 | CaGLP1 |
| 33. | Potato | Solanum tuberosum agglutinin | STA |
| 34. | Pumpkin | Cucurbita maxima agglutinin | CUM |
| 35. | Rice | Oryza sativa agglutinin | OTL |
| 36. | Rye | Secale cereale agglutinin | SCL |
| 37. | Salmon | Salmon serum lectin | SSL |
| 38. | Sesame | Sesamum indicum | SEI |
| 39. | Shallot | Allium ascalonicum agglutinin | AAA |
| 40. | Snail | Helix hortensis agglutinin | HEL |
| 41. | Soybean | Soybean agglutinin | SBA |
| 42. | Squash | Cucurbita pepo agglutinin | CUP |
| 43. | Sweet pea | Lathyrus odoratus agglutinin | LOA |
| 44. | Sweet potato | Ipomoea batatas lectin | IBL |
| 45. | Tomato | Lycopersicon esculentum agglutinin | LEA |
| 46. | Winged bean | Psophocarpus tetragonolobus agglutinin | PTA |
| 47. | Wheat | Triticum vulgare agglutinin | TVA |
| 48. | Wheat germ | Wheat germ agglutinin | WGA |
| 49. | Wheat germ | Succinyl wheat germ agglutinin | sWGA |
| 50. | Wine and grapes | Vitis vinifera lectins | none assigned |
| *Name listed refers to the verbiage which is most commonly seen in medical and scientific papers. A given food can and will have multiple names – e.g. avena sativa agglutinin is the same as oatmeal lectin. **Abbreviation refers to that which is most commonly seen. Some have multiple abbreviations – e.g. SBA and SBL for soy. |
|||
Remember, the above-ranked table is in alphabetical order. Not many of them have had their exact percentages determined, which makes a list ranked by the lowest concentration or potency impossible as of today.
If something does have a higher amount, that does not necessarily means it’s the worse type of food.
For example, the dietary issues with tree nuts tend to revolve around their high phytic acid content and allergy-producing proteins. Most research suggests their lectin content is not pro-inflammatory or causes other effects in humans.
If you want to blame nuts for weight gain, it looks like that will fall squarely on their high fat and calorie characteristics.
You will hear many health bloggers advocating for an anti-lectin or lectin free diet. That’s hilarious because there’s no such thing!
Why? Because these sugar-binding proteins are found in everything you eat, to at least some degree.
Some of those listed above, particularly the grains and milk, are singled out for having greater concentrations, but don’t interpret that as meaning meat, seafood, fish, and non-nightshade fruits and vegetables don’t contain lectins because all those do too!
In short, that makes lectin-free eating impossible, especially since some types are not destroyed by heat or gastric acids (more on that below).
Since we’re talking about an unknown number of different types, you can’t put them all under the same umbrella.
If two foods had a 1% concentration but were different types, would you be able to say they are equal?
Not necessarily.
Some types appear to have no effect on humans, while others may be inflammatory or even deadly.

Ricin is one of the most deadly poisons known to mankind. It is a lectin found in castor beans.
Just an isolated speck of it might be all it takes to kill you.
Literally, a speck on the tip of an umbrella can kill a human.
That’s how Bulgarian dissident Georgi Markov was killed. During the Cold War, he was assassinated with an “accidental” umbrella poke. (32)
Not always bad?
What about their beneficial effects? Any buzz you may hear is that lectins are really bad for you. That their intolerance makes you fat or has inflammatory side effects.
They often fail to mention that depending on the type, they can have a neutral effect – not being bad or good – or even offer benefits for human health (10).
That’s right, and lectins can be healthy and good for you! You can’t paint them all with the same brush.
It’s kind of like people who start with the premise that bacteria are bad. True, many are dangerous to humans, but many are beneficial and actually necessary for our survival. Without your gut flora, you would be unable to digest nutrients and die.
You can’t avoid lectins entirely. Common sense tells us that if their consumption were entirely bad for us, then no one – humans or animals – would have them in literally everything they eat.
Why do they affect us differently?

As a human, if you were designing them for your own body, your goal would probably be to make them harmless for your species but toxic to all other life forms who may want to feed off or eat you.
That would be the best approach to ensure your survival, right? Because whether it’s a bear, a lion, a dog, or even a mosquito… none would want to eat you because they would associate you (humans) as being toxic.
Well, if you designed your lectins that way and each species did the same for theirs, what would be left to eat on this planet?
We would all be toxic to one another!
On the flip side, being edible to everyone is having the pendulum swing too far in the other direction. If the berries on a bush could be eaten by every animal and insect, how would it survive? It might not because it would have a bullseye on it from every species who encountered it.
But let’s say you took that same bush and made the berries toxic to mammals but safe for birds.
Now you have a balance. The life of the bush is protected from most, but its life is extended to some as a food source.
No one can know why God works the way God does, but this theory is the best we can come up with using our very limited human minds.
Different effects within the same species
We’re not all clones of each other.
Within the past several decades, science has gained a much greater understanding of the biological differences within the same species. Differences not believed to exist previously, or thought of as rare genetic mutations, or perhaps fringe theories concocted by rogue minds.
For example, sexuality. Thanks to newer MRI technology, scientists have begun realizing there really is a difference in the brains of straight and gay people. The latter exhibit patterns more comparable to the opposite gender than their own.
Naysayers will claim that different brain pattern is due to what they view as years of deranged and deviant sexual behavior.
Then how do they explain the fact that these brain pattern changes are seen in “gay acting” children (who later in life are gay) even before they go through puberty and before they have any type of sexual experiences or even fantasies of such? That negates the deviant behavior theory since the brain changes can be seen beforehand.

Yes, many people are delusional and simply follow gluten-free diets as a fad without any scientific understanding or medical testing done to diagnose a disease/sensitivity.
But even setting those types of people aside, thanks to more research and testing in recent years, the medical community understands that food allergies are really a bigger problem than previously thought. They’re not just rare disorders. They can have serious consequences in our lives and may worsen – if not be directly responsible for – a number of autoimmune diseases and perhaps others.
How this all relates to lectins is that even more recent than these controversial topics is the concept that, yes, lectins really do seem to affect some people differently than others, possibly be a large degree.
Research is starting to find that some people may be affected by nightshades like potatoes, while others can eat them their entire life without any adverse side effects whatsoever.
Or maybe it’s a vegan who can eat beans in abundance with minimal farts or other symptoms, while some people eat just a smidgen and experience a total digestive disaster.
Why are lectins bad?
As mentioned above, you can think of them as low-level toxins. Just like chemical poisons, how they may (or may not) harm you depends on the type and amount encountered, along with your body’s unique biology.
When harmful, lectins and inflammation go together like two peas in a pod, pun intended.
But the cause of this inflammation can differ depending on whether it’s occurring inside of your GI tract or outside of it (in your bloodstream).

Remember that part we said about foods being edible for some species but having side effects in others?
Humans don’t make the enzyme known as alpha-galactosidase, which is needed to digest when it comes to beans, peas, lentils, and other legumes.
So they ferment in our digestive tract, and that is what’s causing the side effects.
However, many other species, including other mammals, do have the enzymes and can eat them just fine. For us humans, popping a couple of Beano tablets can work wonders at combating gas and bloating since it introduces enzymes to your meal that break down these tough to digest beans.
Due to these missing enzymes, it’s why raw food dieters should avoid most legumes if they’re going to refuse cooking them.
The heat of cooking, at least with legumes, greatly breaks the oligosaccharides. It also destroys lectins. This is why the lectin avoidance diet should really be re-classified as the proper food preparation diet in many scenarios.
Because people are confusing lectin content in beans and the associated side effects when they are largely unrelated. With tomatoes, cooking destroys the content.
In short, the right food prep methods may be just as effective as avoidance.
That’s not to say the irritation and inflammation from lectins in beans can’t be a major problem, though. Certain types and concentrations definitely can be.

To put it in perspective, here’s how red kidney beans test out for their lectin content (11):
- Raw = 20,000 to 70,000 hau (hemagglutinating unit)
- Fully cooked = 200 to 400 hau
That’s a 99% or greater reduction from the heat of cooking. By that simple act, you have turned them into low-lectin food.
This type of bean is by far the worst dietary offender, as even white kidney beans have about 67% less than their red cousins. Broad beans are 90 to 95% less. The content in soy is bad, but it’s easy to neutralize (more on that below).
The side effects of phytohaemagglutinin in kidney beans take place in the GI tract. Or triggering symptoms that are directly caused by nerve stimulation down there (i.e. feeling nauseous).

But you hear people blaming lectin intolerance and toxicity for everything from asthma to rheumatoid arthritis and even conditions of the brain like autism and Alzheimer’s. Things like that are clearly unrelated to the digestive system if these lectins are not being absorbed and therefore remain isolated to the GI tract. Right?
Maybe not.
It is correct that most lectins are quite resistant to stomach acids and the digestive process. For that reason, they were not believed to be absorbed. Meaning they could not enter your bloodstream and flow to other parts of the body (like the brain, kidneys, liver, or joints).
The history of lectins really changed in 1989, when it was first discovered that some of these indigestible proteins are instead permeating through the gut wall and then having free reign to run wild in your blood stream. They can then deposit themselves in distant organs (12) (13).
That may not seem surprising to read, but you need to understand that when something is not absorbed during digestion, it’s typically assumed that it does not enter the bloodstream and therefore is harmless outside of the GI tract.
For example, the heavy metal barium, which you drink for CT scans of the abdomen, is highly toxic.
However, in the form of barium sulfate, which is the form used for these radiological purposes, it is not believed to be absorbed in all and therefore is considered safe because presumably all of it passes through the digestive tract fully intact without entering your bloodstream.
That was how scientists previously thought of the indigestible glycoproteins.
When this discovery came about in 1989 that some indigestible lectins may actually be absorbed and entering the blood stream, it changed the whole paradigm of how we must evaluate them.
Suddenly, the fact that our digestive juices and gut flora don’t break them down does not automatically guarantee their safety. Because they still might be entering our bloodstream through what’s been coined leaky gut.
Having your average dietary sugars “leak” through your intestinal wall is probably harmless. The concern isn’t about the normal things your body would absorb regardless.
The real worry is about stuff getting through which your body doesn’t want. Toxins, heavy metals, and large foreign molecules that your body has no nutritional use for.
How lectins and leaky gut may be related by making the condition worse. Some theorize these glycans may even be triggering it in the first place in an otherwise healthy GI tract which does not have abnormal “holes” in it.
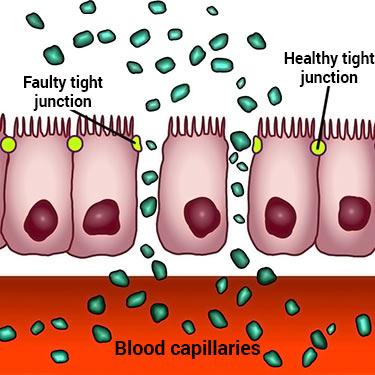
How lectins function to do this by irritating the intestinal lining.
The tight junctures in the lining are normally closed to prevent undesired substances from getting through. After being irritated by some types, it has been found that these junctures of mucosa cells are stimulated to open up – like a door – allowing unrestricted access for things to flow through and go directly into your bloodstream.
It’s this leaky gut syndrome, so there is evidence piling up to suggest lectin irritation might be affecting seemingly unrelated diseases, too.
Diseases caused by lectins
It’s important to reiterate how little is really known about these compounds. There are thousands of different types of lectins in our diet. Among those identified, only a small fraction have been extensively studied.
Furthermore, thousands of complex carbohydrates (sugars) are used by our cells for signaling.
So not only do we not know how many lectin types there are, but to further complicate matters, we have no clue how even the most common ones interact with all the different types of sugars which they may encounter in the body. Some interactions may be harmless, others not.
Due to the many unknowns, no one can say for sure which diseases might be caused by their presence. Given the many different types of these sugar-binding molecules, it’s a bit too generic to say they are a cause in general simply.
It doesn’t mean all lectins are the problem, even if they do. Most likely, it’s a few very specific types, and even with those, they’re probably only affecting some – not all – sufferers of a given disease.
While unproven, there have been accusations that lectins may have a role – either as a direct cause or a worsening factor – in some cases of the following diseases and health conditions:
Wheat lectin vs. Celiac disease

Yes, it is true that diagnosing Celiac or heightened gluten sensitivity is complicated. There’s not really a simple test to do so.
Oftentimes, the diagnosis is based on at least two different food allergy tests.
The most extreme is a biopsy of the small intestine to see how the lining has been affected. Since that’s a surgical procedure, more often, it is blood testing used to check for gluten antibodies and/or a “poop test” where the same is done with your fecal matter.
There is also genetic testing to look for certain alleles which correlate with Celiac disease, but that is not a reliable diagnosis in and of itself.
However it’s diagnosed, only a very small portion of the population – in the low single digits percentage – tests out positive for these. Or, to be more technically accurate, tests positive for having either Celiac or substantially higher elevated gluten antibodies (everybody will have at least some antibodies, for all foods).
The exact percentage is highly disputed, but it is a low number. For the sake of math, let’s say 7% of the population has a gluten allergy/sensitivity, which is a figure that’s sometimes cited (14).
If it is only 7% of the population, then why do three times that number – 21% of Americans – try to “actively include” gluten free in their diet. Many of them claim it makes them feel better and helps with weight loss.
That 21% is the average of 19% for men and 23% for women, according to a Gallup poll (15).
Is it because the other 14% are uninformed?
Is it because of the placebo effect?
The answer to both is yes, to at least some degree.
But within that 14%, there is evidence to suggest legitimate reasons why they might experience real improvement in their health, even though their gluten sensitivity is completely normal.
Brain fog from lectins is a common theme you will hear, but the truth of the matter is that even if they’re entering through a leaky gut, that doesn’t necessarily mean they can get into your brain. Your blood-brain barrier (BBB) blocks many things, primarily large molecules.
To get through, large molecules need shuttle-mediated transport (something to latch onto, which can permeate the barrier).
The mass for one type of chicken lectin was found to be 69,000 daltons (16). For tomatoes, one is 65,633 (17). Phytohemagglutinin-L (PHA-L) isolated from the red kidney bean has a weight of approximately 120,000 (18).
Leeks and taro are foods with mannose-binding lectin (MBL), often high molecular weights.
In humans, 600,000 daltons are what one particular lectin specific to mannose and N-acetylglucosamine residue tested out at. A smaller sub-unit of it was still 31,000 (19).
How big can they be to enter the brain?
400 to 600 daltons are considered the max for being able to penetrate the BBB (20). While some scenarios allow for larger, you’re not talking tens of thousands of daltons.
Wheat germ agglutinin (WGA) is what discourages insects and bacteria from eating the wheat kernal. That’s the size of 38,000 daltons (21).
So how would wheat lectins, which are 38,000 daltons, get through the blood-brain barrier, which only allows up to around 600?
That’s not exactly like squeezing into a tight pair of jeans. You need a magic trick here because if you’re a WGA lectin, it’s like having a butt that is 60x bigger than the opening of the doorway.
Well, there might be a magic trick that can make that happen.
In 1988 it was discovered that wheat lectins could pass through the blood brain barrier of mice and rats through what’s called adsorptive endocytosis.
That was discovered by the University of Maryland (22). In the 30 years since, there’s been almost no research involving wheat germ agglutinin and adsorptive endocytosis. So whether this happens in humans appears to be unknown, and obviously, it’s not easy to measure without opening a human brain or using things like radioactive tracers, which no one in their right mind would voluntarily do.
While there is some evidence that dairy can do the same, when it comes to foods, Dr. William Davis claims that wheat is unique in its ability to cross the BBB.
Dr. David talks about this in his #1 New York Times bestseller, Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health.
If that theory is true, maybe that’s why such a large number of people report less brain fog and other Celiac-like symptoms when they go gluten-free.
Ultimately, research on how wheat lectins and leaky gut might affect the brain is only beginning.
Before you go on a wheat-free diet, it is worth noting that research has found that WGA may actually enhance the natural anti-cancer (tumoricidal) activity of human monocytes (a type of white blood cell). This was seen with select types of cell lines for bladder cancer, melanoma, kidney cancer, and glioblastoma (brain cancer), which were tested in the lab (23).
Like anything, there are pros and cons to consider.
Lectin avoidance diet?
This is one of those topics where both sides need to be humble and admit they don’t know what they don’t know.
The naysayers will call this avoidance diet a hoax, making the argument that foods containing lectins have been part of human history from the get-go.
That is true, but in what quantity?
In the hunter-gatherer days, perhaps a few kernels of wheat, barley, or other grain would be picked by hand. But given the labor involved, you can bet they weren’t depending on it as a staple of their diet.
When milling and the production of flour came about, it was desired to discard the bran (outer shell) of the grain and make the flour using what’s inside. Therefore, much of the grain lectin was discarded.
That’s why the amount of lectin in white rice is lower than in brown rice.
Where did peasant bread get its name? Here’s a clue, it wasn’t made using white flour – that is the most labor-intensive and expensive form to produce.

Could this shift have anything to do with the seemingly increasing rates of food allergies OR symptoms masquerading as such, which might be caused by lectins in grains?
You can’t say for sure one way or another.
For that reason, if you are experiencing health issues, it certainly wouldn’t hurt to try a lectin diet. That’s where you selectively remove a type – like nightshades – and see how you do over the period of a few weeks without it.
Then rinse and repeat with other types. You might find you are intolerant to certain types.
But as we said above, lectin-free foods are a misnomer. You can’t remove them entirely, but learning how to reduce lectins in food is a viable diet plan.
If they’re in everything, where do you begin?
Great question.
Just because a given food might have more of them does not automatically mean it’s a problem.
This is because there are thousands of different types in your diet, and their effects on the human body can vary greatly from lethal poisoning like ricin to having no effect at all.
With that caveat said, based on the limited knowledge known today, even though they’re not apples to apples, it does seem logical to start by reducing foods that contain lectins in high amounts.
Sure, if there was a 1% concentration in a milk product and a 1% concentration in a wheat product, that doesn’t mean they’re equal in effect. Nor does it mean they’re better than a food with a 2% concentration of a different type.
In an ideal scenario, you would be able to weigh each type accordingly. But with the exception of a few specific foods like kidney beans, any health effects they may have is poorly understood. For that reason, starting with the elimination of the highest-known sources isn’t a bad idea.
How to reduce or remove lectins
Rather than avoiding these foods entirely, the preparation method is arguably far more important.
Though for some types of foods, cutting them out entirely may be the only effective method for real reduction.
Potatoes

Whether it’s yams, sweet potatoes, purple potatoes, potato starch, or any other form… they’re all going to contain high amounts of glycan-binding proteins. However, the sweet varieties are not part of the nightshade family.
The potato lectins are very stable under heat, acid, and base solutions. This is believed to be due to its high carbohydrate content and the disulfide bonds formed with them.
The STA can be deactivated under oxidative conditions and is “easily inactivated” by sodium hydrogen sulfite, which is also known as sodium bisulfite (29).
That chemical was a food additive, but for use on fruits and vegetables, the FDA banned it in 1986 (except for making wine and beer).
It was banned because it killed 13 people by triggering asthma attacks and injuring countless others. (30). That rules out its use as a method for reducing lectins in potatoes.
As far as oxidative conditions which might deactivate them, many cooking methods fall under that category. However, the baking and frying of potatoes will produce high amounts of acrylamide, a class 2A carcinogen, according to the World Health Organization.
Dairy

However, we live in a country where buying or selling unpasteurized milk is a criminal offense in many scenarios. Even if you got your hands on it, there would still be a high amount of lectins in milk, regardless.
The suggestion that grass-fed milk, butter, or ghee contains less is not something that is backed by studies.
This is why forgoing dairy altogether for a while and seeing how you do is a good strategy. Aside from milk, that also means no butter, cheese, ice cream, or other derivatives during your test.
Vegan milk is made with nuts and oils that generally have low lectin, or their forms have little to no apparent effect in humans. Almond milk, cashew, and other nuts appear to be safe.
Intolerance from coconut oil and others used to make them won’t be a problem either since refined oils are pure fats (not proteins).
Be aware of how many almonds are in the milk because it’s not many!
Grains

Later research reportedly detected heat-stable lectin activity in gluten from gliadin and the acid-soluble gluten fractions of gluten. This was different from the activity coming from wheat germ, as cross-contamination was ruled out (25).
Whether it’s gluten grains like wheat, barley, and rye, or GF alternatives like oats, sorghum, and millet, you may want to try switching to baked goods and cereals made with more refined flours, not whole grains or bran since those contain high amounts of lectin. There will be an adverse glycemic effect in doing so, so consult your doctor beforehand if you are a type 1 or type 2 diabetic.
White rice is also high glycemic, but it contains fewer glycoproteins when compared to brown rice.
Does quinoa have lectins? Of course, but they have not yet been studied. There isn’t even a designated abbreviation for quinoa agglutinin.
Being a gluten-free seed rather than a grain, it’s possible that quinoa might have an effect similar to other flowering seeds like sunflower, flax, and hemp. Those have no obvious impact on humans. Allergies to hemp are another story.
Like quinoa, buckwheat is another gluten-free pseudo-grain. It’s related to rhubarb. It might be another low-lectin food, or more accurately said, one which is less likely to have dietary side effects when compared to gluten, gliadin, and wheat germ agglutinin. But with no published research on buckwheat lectin, any advantage it may offer non-Celiacs for digestive tolerance is speculative.
Legumes

How to remove lectins from beans involves soaking them overnight, rinsing and draining them, then thoroughly cooking them. They are not something you want to undercook!
If you don’t have the time to soak canned beans overnight in the fridge, that’s fine, but just make sure you rinse them well before cooking.
The heat will almost entirely destroy lectins in beans, as pointed out in the numbers above for kidneys. You can deactivate lupin, fava, and soybean lectins entirely at 100° C (212° F) for at least 10 minutes (26).
With lower temperatures, that’s not the case. Cooking legumes at 70° C (158° F) for several hours “has little or no effect” (27).
Soy milk is heated (pasteurized) but is that for at least 10 minutes? The manufacturers don’t say. For that reason, veer on the side of caution and avoid soy milk if you are worried there might be active agglutinins in it.
Peanuts are actually a legume. Since this “nut” is always sold roasted, a lower amount of peanut agglutinin (PNA) would be expected.
Try sprouting them before cooking if you’re using prepared fresh or dry beans/lentils. The germination process further reduces the amount they contain.
Raw coffee beans will contain large amounts. Considering how thoroughly those beans are heated – probably more than any other food or drink in your diet – your cup of joe is likely quite safe. Coffee has not been researched through pre and post-roasting.
Tomatoes

There is a rumor that tomatoes’ lectins are enhanced with the heat of cooking.
That’s not true.
It’s actually the antioxidant lycopene that has enhanced bioavailability from heating (31).
Since they’re often eaten raw in salads, tomato lycopersicon esculentum lectin (LEL) often bypasses any heat, and for that reason, it has been estimated that the average American consumes 200 mg per year of it.
Now you have two good reasons to pig out on that plate of spaghetti marinara. Maybe even three if you’re using GF pasta made with refined flour. But do please cook those noodles extra al dente (extra firm to the bite, which is slightly undercooked). That can help lower the glycemic impact.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.